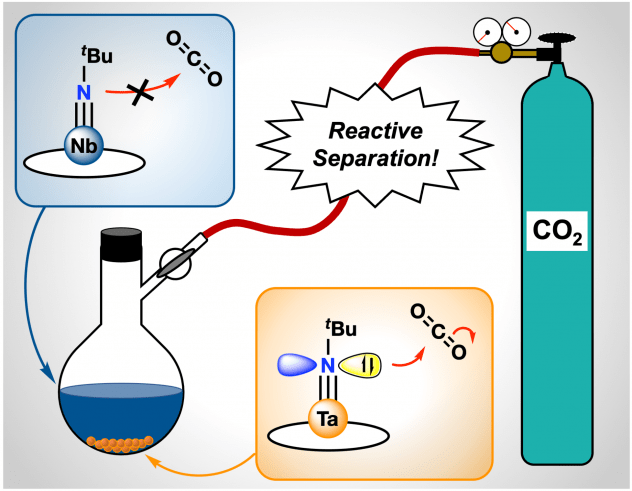
Congratulations to Alex Weberg, Subha Chaudhuri, and Christian Uruburo and colleagues in the Schatz and Schelter groups on their newest paper, titled “Tantalum, Easy as Pi: Understanding Differences in Metal-Imido Bonding Towards Improving Ta/Nb Separations”! In this paper, we introduce new Ta/Nb imido compounds: M(tBuN)(TriNOx) (1-M) bound by the TriNOx3– ligand and demonstrate a fundamental, proof-of-concept Ta/Nb separation based on differences in the imido reactivities. Despite the nearly identical structures of 1-M, density functional theory (DFT)-computed electronic structures of 1-M indicate enhanced basic character of the imido group in 1-Ta as compared to 1-Nb. Accordingly, the rate of CO2 insertion into the M=Nimido bond of 1-Ta to form a carbamate complex (2-Ta) was selective compared to the analogous, unobserved reaction with 1-Nb. Differences in solubility between the imido and carbamate complexes allowed for separation of the carbamate complex, and led to an efficient Ta/Nb separation (STa/Nb= 404 ± 150) dependent on the kinetic differences in nucleophilicities between the imido moieties in 1-Ta and 1-Nb. This methodology represents an alternative approach to current industrial processes of Ta/Nb that are both energy intensive and occur in caustic and toxic conditions.