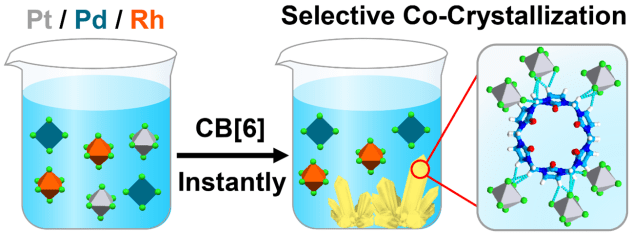
Selective Separation of Hexachloroplatinate(IV) Dianions Based on Exo-Binding with Cucurbit[6]uril
We report the instantaneous co-crystallization and concomitant co-precipitation between [PtCl6]2− dianions and cucurbit[6]uril, a phenomenon which relies on the selective recognition of these dianions through noncovalent bonding interactions on the outer surface of cucurbit[6]uril. The selective [PtCl6]2− dianion recognition is driven by the weak [Pt-Cl×××H-C] hydrogen-bonding and [Pt-Cl×××CO] ion-dipole interactions. The synthetic protocol is highly selective. It is not observed in combinations between cucurbit[6]uril and other Pt- and Pd- or Rh-based chloride anions. We have also demonstrated that cucurbit[6]uril is able to separate selectively [PtCl6]2− dianions from a mixture of [PtCl6]2−, [PdCl4]2− and [RhCl6]3− This highly selective and fast co-crystallization protocol, in principle, could be exploited to recover platinum from spent vehicular three-way catalytic converters and other platinum-bearing metal waste.
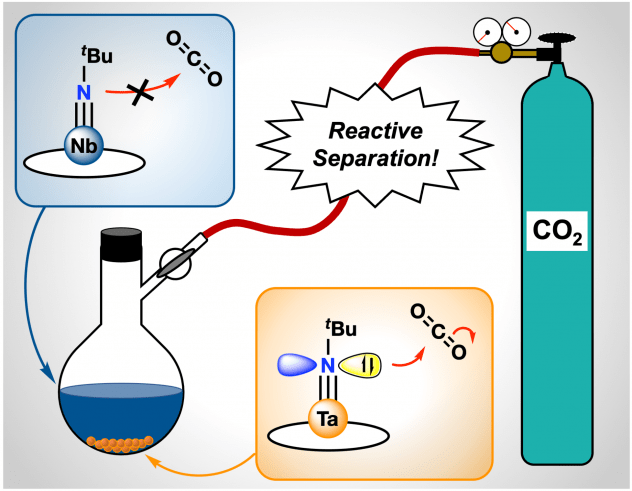
Tantalum, Easy as Pi: Understanding Differences in Metal-Imido Bonding Towards Improving Ta/Nb Separations
The Schatz and Schelter groups have developed proof-of-concept methods for the separation and purification of niobium and tantalum, which co-occur in natural sources, is difficult due to their similar physical and chemical properties. The current industrial method for separating Ta/Nb mixtures uses an energy-intensive process with caustic and toxic conditions. It is of interest to develop alternative, fundamental methodologies for purification of these technologically important metals that improve upon their environmental impact. Herein, we introduce new Ta/Nb imido compounds: M(tBuN)(TriNOx) (1-M) bound by the TriNOx3– ligand and demonstrate a fundamental, proof-of-concept Ta/Nb separation based on differences in the imido reactivities. Despite the nearly identical structures of 1-M, density functional theory (DFT)-computed electronic structures of 1-M indicate enhanced basic character of the imido group in 1-Ta as compared to 1-Nb. Accordingly, the rate of CO2 insertion into the M=Nimido bond of 1-Ta to form a carbamate complex (2-Ta) was selective compared to the analogous, unobserved reaction with 1-Nb. Differences in solubility between the imido and carbamate complexes allowed for separation of the carbamate complex, and led to an efficient Ta/Nb separation (STa/Nb= 404 ± 150) dependent on the kinetic differences in nucleophilicities between the imido moieties in 1-Ta and 1-Nb. Results were published here.
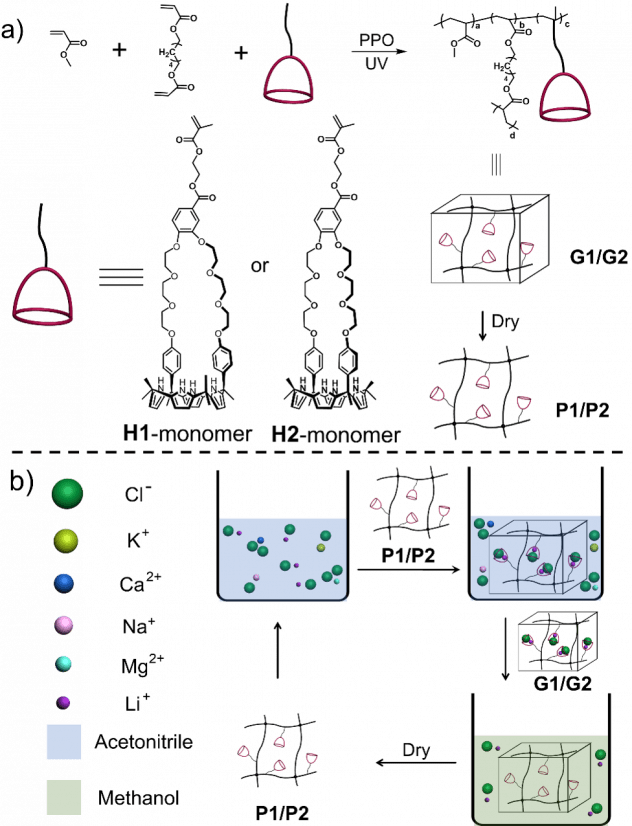
Selective Separation of Lithium Chloride by Organogels
The groups of Schatz (Northwestern University), Page, and Sessler (Univ of Texas-Austin) have found new methods for selective separation of lithium using organogels containing strapped calix[4]pyrroles. Lithium and its compounds play critical roles in high-energy batteries, nuclear fusion power generation and ultra-light and high-strength lithium alloys. Currently, lithium is obtained primarily from minerals and brines, as well as to a much lesser extent via the recycling of lithium-containing waste. In these sources, lithium typically coexists with various metal salts (such as those of sodium, potassium, magnesium, and calcium). The similarity between the associated salts (e.g., NaCl vs LiCl) makes the selective extraction of lithium a challenge. Therefore, the development of new methods for purifying lithium constitutes an important research direction.
We report two functionalized crown-ether strapped calix[4]pyrroles. They were introduced into a cross-linked acrylate polymer system to give two organic gels that allowed for the selective extraction of lithium chloride from acetonitrile and its subsequent release into methanol.
Manipulating Nuclear Dynamics Through Electron Spin and Vice Versa
Yanze Wu and Joseph E. Subotnik from the University of Pennsylvania report that when molecular dynamics pass near a conical intersection, even a small amount of spin-orbit coupling can lead to a huge Berry force effect, which dramatically changes the pathway selection. In particular, for a simple radical reaction with two outgoing reaction channels, an exact quantum scattering solution in two dimensions shows that the presence of a significant Berry force can lead to spin selectivity as large as 100%. The results were published in Nature Communications (link).
A reaction channel where spin dictates choice of product.
Electronic spin is one of the most fundamental and profound observables in quantum mechanics, and spin manipulation is an important topic today with various real-life applications, for example, magnetic data storage. We contend the spin polarization originating from nuclear dynamics should have broad implications for a wide range of phenomena, including novel chirality-induced spin selectivity effects, magneto-chemical metal separations reactions, or even organic spintronic devices.
The research was funded by the Center for Sustainable Separations of Metals (CSSM), a National Science Foundation (NSF) Center for Chemical Innovation (CCI), grant number CHE-1925708. This research was also supported by University of Pennsylvania.
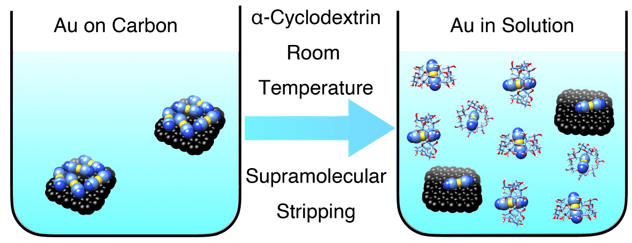
Supramolecular Gold Stripping from Activated Carbon at Room Temperature Using α-Cyclodextrin
A team from Northwestern University consisting of Wenqi Liu, Leighton O. Jones, Huang Wu, George C. Schatz, and J. Fraser Stoddart reports the molecular recognition of the Au(CN)2- anion, a crucial intermediate in today’s gold mining industry, by α-cyclodextrin in the presence of potassium (K+) ions. They demonstrate that this molecular recognition process can be applied to the stripping of gold from the surface of activated carbon at room temperature. Moreover, this stripping process is selective for Au(CN)2− in the presence of Ag(CN)2−, which has a lower binding affinity toward α-cyclodextrin. The results were reported in the Journal of the American Chemical Society (link). The work was also featured as front cover artwork in the journal. 
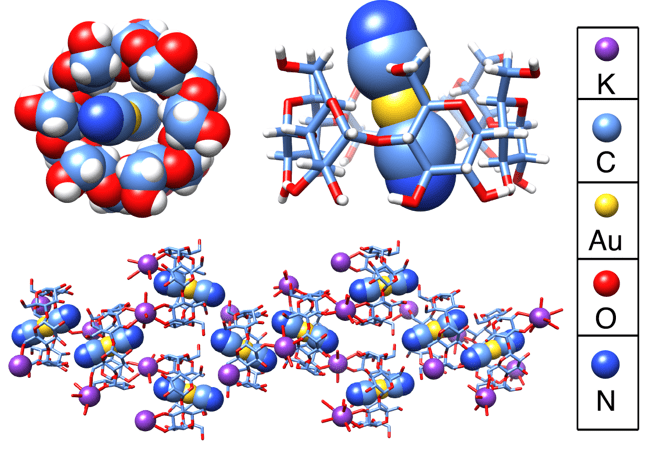
Space-filling and tubular representations of the solid-state superstructures of KAu(CN)2⊂α-CD obtained from single-crystal X-ray diffraction studies.
In today’s gold-mining industry, in order to strip the dicyanoaurate salts from the activated carbon, harsh conditions, including high temperatures (95−140 °C), high pressures (70−400 kbar), and concentrated cyanide and hydroxide solutions, are required. The molecular recognition reported as a result of our research, could, in principle, be integrated into commercial gold-mining protocols and lead to significantly reduced costs, energy consumption, and environmental impact.
Graphical illustration of gold stripping from the surface of activated carbon into aqueous solution using α-cyclodextrin.
The research was funded by the Center for Sustainable Separations of Metals (CSSM), a National Science Foundation (NSF) Center for Chemical Innovation (CCI), grant number CHE-1925708. This research was also supported by Northwestern University.
High-Efficiency Gold Recovery using Cucurbit[6]uril
A team from Northwestern University consisting of Huang Wu, Leighton O. Jones, George C. Shatz, and J. Fraser Stoddart reports a highly efficient gold-recovery protocol on the basis of the instantaneous assembly between a macrocycle, cucurbit[6]uril (CB[6]), and gold-bearing salts, MAuX4 (M = H / K & X = Cl / Br) anions. A laboratory-scale gold-recovery process has been established based on the co-precipitation of CB[6]×HAuCl4, in which 99.8% of the gold present in the raw material has been recovered. The results were reported in ACS Applied Materials and Interfaces (link).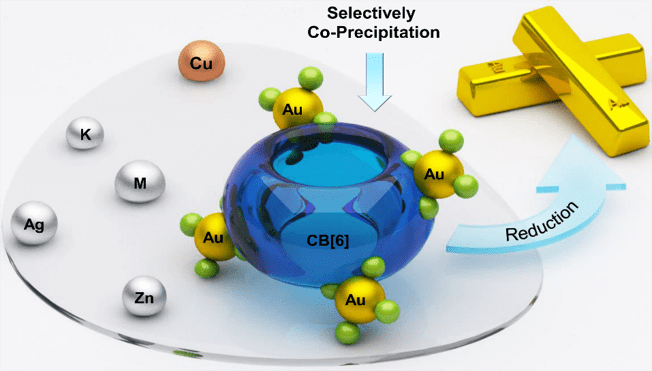
In our electronic age, the disposal of waste draws more and more attention worldwide. If not handled wisely, the toxic composition of this waste becomes hazardous to the environment and to humankind. This electronic waste, however, can become a valuable resource since it contains many noble metals and other useful materials. Gold plays an indispensable role in today’s electronics industry. About 300 metric tons of gold are used in electronics manufacture every year. The recovery of gold from electronic waste is important from economic and environmental perspectives.
The research was funded by the Center for Sustainable Separations of Metals (CSSM), a National Science Foundation (NSF) Center for Chemical Innovation (CCI), grant number CHE-1925708. This research was also supported by Northwestern University.