
A team from Northwestern University consisting of Wenqi Liu, Leighton O. Jones, Huang Wu, George C. Schatz, and J. Fraser Stoddart reports the molecular recognition of the Au(CN)2- anion, a crucial intermediate in today’s gold mining industry, by α-cyclodextrin in the presence of potassium (K+) ions. They demonstrate that this molecular recognition process can be applied to the stripping of gold from the surface of activated carbon at room temperature. Moreover, this stripping process is selective for Au(CN)2− in the presence of Ag(CN)2−, which has a lower binding affinity toward α-cyclodextrin. The results were reported in the Journal of the American Chemical Society (link).
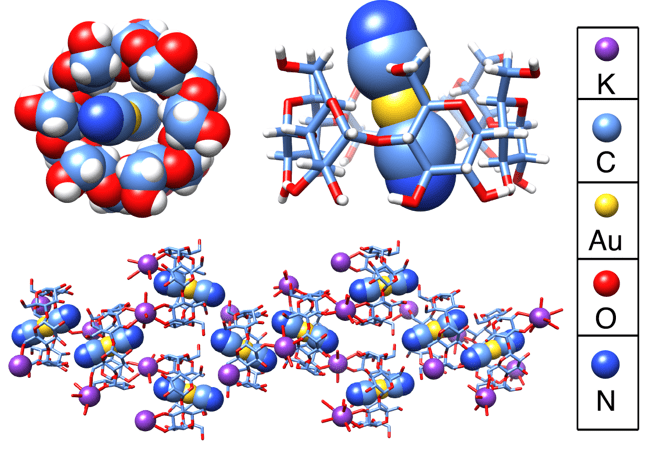
Space-filling and tubular representations of the solid-state superstructures of KAu(CN)2⊂α-CD obtained from single-crystal X-ray diffraction studies.
In today’s gold-mining industry, in order to strip the dicyanoaurate salts from the activated carbon, harsh conditions, including high temperatures (95−140 °C), high pressures (70−400 kbar), and concentrated cyanide and hydroxide solutions, are required. The molecular recognition reported as a result of our research, could, in principle, be integrated into commercial gold-mining protocols and lead to significantly reduced costs, energy consumption, and environmental impact.
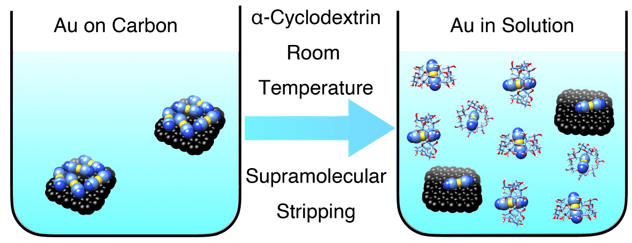
Graphical illustration of gold stripping from the surface of activated carbon into aqueous solution using α-cyclodextrin.
The research was funded by the Center for Sustainable Separations of Metals (CSSM), a National Science Foundation (NSF) Center for Chemical Innovation (CCI), grant number CHE-1925708. This research was also supported by Northwestern University.
